Parkinson’s Disease
Disease Background
Parkinson’s disease (PD) is characterized by chronic neurodegeneration in the midbrain region of the brain, affecting mainly dopaminergic neurons.

Greater than 500,000 Americans suffer from this progressive, debilitating disease. Current therapies only affect some of the symptoms, and there are no therapies that reverse or halt the motor dysfunction due to neuron loss.
Unfortunately, many Parkinson’s patients also suffer from cognitive disabilities as the disease progresses. Mixed results have been found with transplantation of fetal neural stem cells into the brains of PD patients. Originally introduced in the late 80s, serious side effects were noted, including dyskinesia following implantation of stem cells.
Other invasive, growth factor therapeutic approaches have been attempted, but also with little success and with increased safety risks.
Overview of Neuronascent’s Lead Parkinson’s Disease Candidates
Neuronascent has discovered non-invasive (oral, with no need for brain injection) therapeutic candidates that are neuron regenerative. In other words, the therapies aim to promote the proliferation, differentiation and ensure the survival of nascent neurons in the midbrain region, an area where neurons are selectively lost in Parkinson’s disease.
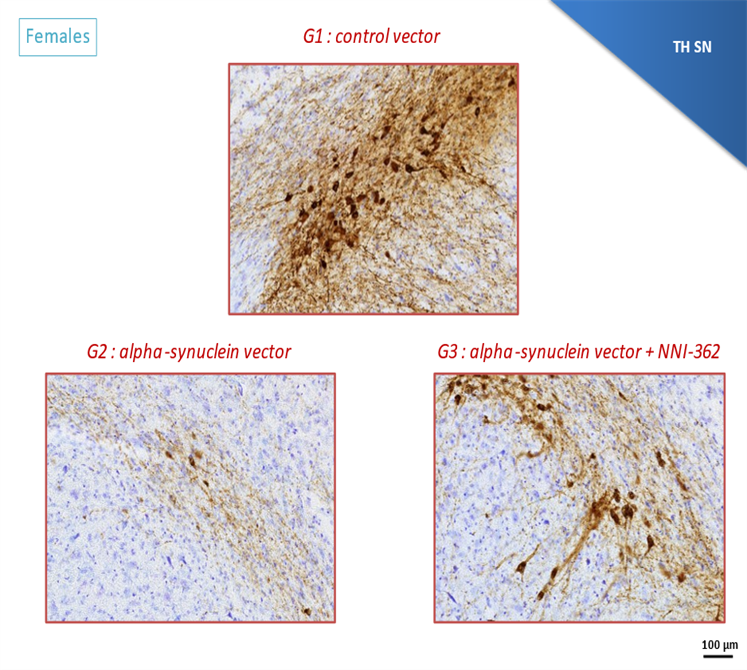
Our lead candidate for Alzheimer’s disease, NNI-362 that is effective in promoting new neurons in a number of disease models, was shown to halt all further dopaminergic connection loss in a pilot model of chronic, progressive Parkinson’s disease. NNI-362 treatment even demonstrated a trend toward regeneration of neuron connections in the caudate putamen (see graph) and at the same time increased the number of new neuronal progenitors, suggesting neuron regeneration, not just neuroprotection. A further model of progressive Parkinson’s disease, the AAV alpha synuclein rat model, showed true regeneration of dopaminergic neurons in the substantia nigra, a selective region of neuron loss in PD. This suggests there is a strong rationale to test NNI-362 in a Phase 2 Parkinson’s disease trial as well as Alzheimer’s disease.
Neuronascent’s back-up therapeutic candidate, NNI-370 was optimized from a completely separate and novel small molecule family. It represents an exciting new class of potential therapies, that in vitro promotes new neuron growth from ventral midbrain stem cells and that inhibits neuron loss due to key initiators of dopaminergic neuron death. This patented therapeutic is now in the preclinical testing phase.
Pre-clinical models of NNI-362 in chronic progressive model of Parkinson’s disease.
- NNI-362 administered for just over 2 weeks showed complete inhibition of all further neuron terminal loss , but also showed a trend for neuron regeneration as had been seen for other disease models. All work was completed in Dr. Charles Meshul’s laboratory at the VA in Portland Oregon.
- AAV alpha synuclein model (Motac, France) Dopaminergic (Th+) increased due to NNI-362 chronic once daily oral treatment, following AAV alpha synuclein vector that promotes degeneration and halts new neuron growth.