Alzheimer’s Disease
Disease Background
Alzheimer’s disease (AD) is the most common form of dementia, which progressively worsens with age. Though individuals can live with the disease for up to 20 years, it is now thought to be the 6th leading cause of death in the US. Presently, 5.6 million Americans suffer from idiopathic Alzheimer’s disease and that number may top 13.2 million by 2050. This devastating disorder may begin as a mild form of new memory loss, but progresses to the point that the patient needs help with basic daily functions. Though caregivers provide 17 billion unpaid hours, eventually, in the later stages, patients are often placed in facilities that can provide 24 hour a day assistance.
Please view the Alzheimer’s Association’s 2019 Facts and Figures about Alzheimer’s disease using the following link: https://alz.org/media/documents/alzheimers-facts-and-figures-2019-r.pdf
Unfortunately, there are no cures for the disease and the present marketed AD therapies moderately reduce the symptoms of the disease, without reversing or halting the cognitive and neurogenesis deficits.
Overview of Neuronascent’s Clinical-Stage Alzheimer’s Disease Therapeutic – NNI-362
Neuronascent has discovered a novel class of therapeutics, called Neuron Regenerative Therapies, that aim to reverse the cognitive deficit in AD patients, by stimulating new neurons to maturation and protecting these “nascent” neurons from further neurodegeneration. See our published article Sumien et al., 2021.
Through painstaking optimization of our patented family of neuron regenerative synthetic therapies, a lead candidate emerged, NNI-362. Neuronascent has now: 1) Completed pre-clinical efficacy testing and IND-enabling safety testing and manufacturing, 2) submitted IND application and received clearance from the FDA for first-in-human clinical trial, and 4) completed Phase Ia human safety testing in healthy aged population. All these milestones have been achieved with the support of the National Institute on Aging (RO1 AG056561). Now Neuronascent is moving NNI-362 toward Phase 2 proof-of-concept trials in mild to moderate Alzheimer’s patients.
Animal Model of NNI-362 Efficacy
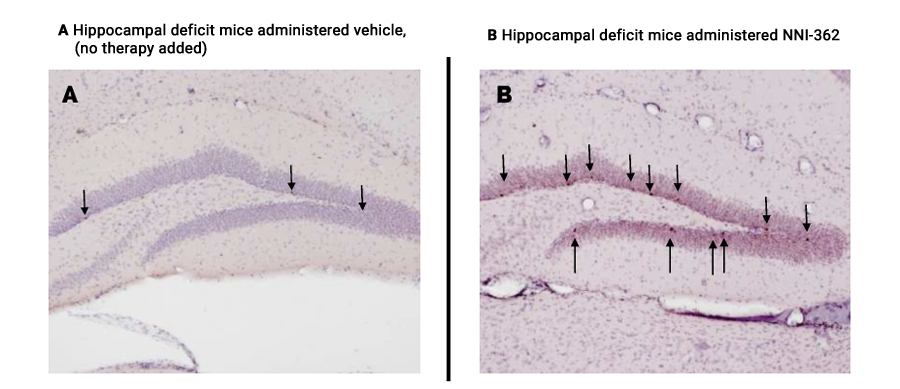
In vivo testing of Neuronascent’s patented, orally-active therapeutic demonstrated that NNI-362 significantly increased the number of adult-born neurons in a key memory region of the brain, the hippocampus. These neurons are associated with the actual reversal of memory impairment in elderly mice. In other words, aged mice receiving daily oral administration of NNI-362 were no different than young animals in formation of healthy new neurons and in their cognitive behavior. A similar reversal of both cognitive impairment and neuron formation in the brain, back to wild type or normal animal levels was observed using another well-established hippocampal deficit mouse model, the Ts65Dn Down syndrome mouse model.
Figures A & B
Greater Numbers of New Neurons in the Hippocampus of Hippocampal Deficit-Modeled Mice Survive to Maturity and Functionality Following NNI-362 Administration (arrows point to new cells in neuron-functioning region)
Summary
These brain-changing events, initiated by NNI-362’s first-in-class allosteric mechanism-of-action, should provide more than just symptomatic relief to AD patients already suffering cognitive deficits. With NNI-362’s clean safety profile to date and a clear path forward, Neuronascent aims to continue from first-in-human safety trials, to proof-of- concept disease-modifying trials of mild to moderate Alzheimer’s patients. While a number of pharmaceutical companies look at earlier and earlier stages of the disease, Neuronascent sees NNI-362 as potentially helping the millions of patients already suffering from this debilitating memory disease of the very aged.
