Veterinary Medicine Program
Neuronascent (NNI) Receives IACUC Approval to Begin Treatment of Aged Dogs with Probable Canine Cognitive Dysfunction (CCD) in Fairbanks, Alaska Using NNI’s Novel Neuron Regenerative Therapy – Cajuvenate
Neuronascent and the University of Alaska – Fairbanks (UAF) are working together to test and treat older sled dogs and canine pets that have probable Canine Cognitive Dysfunction (CCD) a similar disorder to Alzheimer’s disease observed in humans. The study has been approved by a UAF ethics board and will begin to identify and sign up owners with their aged dogs that have significant behavioral issues. Once identified the owners will work with their own veterinarian and potentially be given the Cajuvenate therapeutic for daily oral administration for up to a 6-month period. Cajuvenate, a novel therapy discovered at Neuronascent has already shown ability in several rodent models to promote new neurons in aged and diseased brains that then reverse the behavioral deficits back to young and normal rodents. The therapeutic, already shown safe in many species will be administered at the lowest efficacious dose. This trial to begin in Alaska may appear to be in reverse order, for normally we cure human’s first, but with the extensive time, cost and regulatory hurdles of human efficacy trials, now we will attempt to cure our “best friends” first.
Cajuvenate
Cajuvenate is a safe and novel oral therapeutic for Canine Cognitive Disorder (CCD) in aged dogs. CCD in aged companion dogs, shares a multitude of disease phenotypes with Alzheimer’s Disease (AD) (Ehrenzweig and Hunter, 2023), thus providing early potentially conclusive proof-of-concept one for the other disorder. NNI-362 (i.e., veterinary use name — Cajuvenate), has already been shown to be safe in 6-month GLP dog safety studies (up to 50mg/kg, oral), and has a clean safety profile in aged humans at 240 mg as a top multi-dose (Phase 1a). In aged humans, not only does NNI-362 appear safe, but significantly reduces the plasma phosphoTau AD biomarker over the multi-dose period, while no change occurred in placebo-treated individuals. NNI-362 diminished levels of phosphoTau hyperphosphorylation also in human brain cultures, so we now plan to determine if Cajuvenate reduces phosphoTau hyperphosphorylation in CCD, which may be responsible for cognitive deficits in aged CCD dogs (Habiba et al., 2021; Hines et al., 2023). In human central nervous system disorders and in CCD aged dogs (Polgar et al., 2016) there occurs a loss of smell, (i.e., anosmia) potentially due to nascent neurons being “appropriated” to the regions of brain neurodegeneration (Kandasamy et al., 2015) and away from the olfactory bulb. This readout is appropriate in both humans and dogs since anosmia may be associated with numerous functional behavior deficits. The cognitive impairments of learning, memory, and anxiety are observed in the aged CCD dogs and measured through a CADES questionnaire that can be filled out by veterinarians and/or dog owners (Madari et al., 2015). Finally, the human dog changes in hippocampal volume can equally be measured using vMRI, where in both AD and CCD the volume loss correlates with cognitive deficits. Following discussions with the FDA’s Center for Veterinary Medicine and beginning the process toward Conditional Approval of Cajuvenate, a placebo-controlled randomized study will then commence to test Cajuvenate in aged companion dogs with probable CCD. This proof-of-concept trial will assess whether Cajuvenate can halt or reverse cognitive and functional deficits to provide a longer and healthier life for our companion dogs. The results from this trial could also benefit the trial design and expectation of outcome in a Phase 2 trial of NNI-362 in mild to moderate AD.
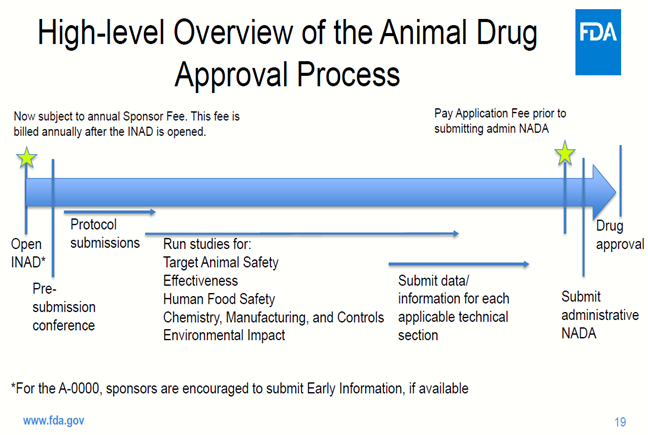
The FDA approval process for Cajuvenate is much faster and cheaper than testing in humans. The process is shown below. Note Neuronascent has requested a waiver for annual Sponsor fees. A protocol has been written the Safety, Chemistry, Manufacturing and Controls sections have already been completed.
New Grant To Halt and Reverse Dementia in Dogs First
CHICAGO, Jan. 8, 2026 — Alzheimer’s Association Part the Cloud today announced more than $11 million in new investments to advance transformational therapeutics for Alzheimer’s and other diseases that cause dementia. Part the Cloud is a global movement with a recognized name and impact that has generated more than $90 million and funded 83 clinical studies of novel potential treatments.
Catalyzing the expansion of the clinical trials pipeline for Alzheimer’s treatments has never been more essential. For more than a decade, Part the Cloud has led the vanguard of investments in new treatment approaches and accelerating the pace of the clinical trials pipeline..
In 2012, Part the Cloud was started by philanthropist Michaela “Mikey” Hoag, whose visionary leadership provided urgently needed funding to fill gaps in support for early to mid-phase clinical trials. Part the Cloud’s funding model emphasizes groundbreaking high-risk, high-reward projects.
There is no other funding mechanism with the scope and breadth of Part the Cloud. Its proven success is demonstrated by the more than $1.6 billion in follow-on funding from the federal government, venture capital firms and other sources. Part the Cloud is specifically designed to speed up translation of experimental treatments from the laboratory into high-quality studies in people.
“Today we are in an era of unprecedented promise in the race to end Alzheimer’s and other diseases that cause dementia,” said Hoag. “I am proud to champion Part the Cloud and its investment into these trailblazing projects as they push to speed up breakthroughs and transform care.”
“Because of Part the Cloud, we are generating real international scientific progress in new potential treatments,” said Maria C. Carrillo, Ph.D., Alzheimer’s Association chief science officer and medical affairs lead. “Now more than ever, it is crucial to support forward-looking projects that can generate hope for the future. This round of Part the Cloud awards catalyzes dynamic projects across three focus areas: early phase clinical trials evaluating novel or repurposed medications or combinations; early phase trials targeting the genetics of Alzheimer’s; and the funding essential to advance the preclinical work to first-in-human clinical trials.”
Each of the 11 grants is for approximately $1 million. The new Part the Cloud grant awardees are:
Translational Research Grant Program
- Glenn Larsen, Ph.D., Aquinnah Pharmaceuticals, Inc. Tauopathy Therapeutic AQV-8741: A Phase I Study.
- Terry Goldberg, Ph.D., Research Foundation for Mental Hygiene, Inc. at New York State Psychiatric Institute. Vortioxetine to improve synaptic connectivity.
- Luis Oskar Soto-Rojas, Ph.D., National Autonomous University of Mexico. Creatine-Augmented Exercise for Neuroprotection in Early AD patients.
- Timothy Siegert, Ph.D., Allyx Therapeutics, Inc. A Phase 2A Trial of ALX-001 (BMS-984923).
- Alireza Faridar, M.D., The Methodist Hospital Research Institute. IL-2 plus GLP-1 RA Combination Therapy to Target Inflammation in AD.
Gene Targeting Challenge
- Rita Balice-Gordon, Ph.D., Muna Therapeutics. Small Molecule TREM2 Agonism: Phase 1 and Observational Studies in Early AD.
Enable the Molecule Program
- Evan Lebois, Ph.D., Violet Therapeutics, Inc. Novel small molecule EPHB3 inhibitors to prevent AD synapse loss.
- Anindya Bhattacharya, Ph.D., Switch Therapeutics, Inc. Targeting MAPT knockdown by a unique siRNA platform to treat Alzheimer’s.
- Can Zhang, M.D., Ph.D., and Changning Wang, Ph.D., Massachusetts General Hospital. Discovery of novel epigenetic inhibitors for Alzheimer’s disease.
- Mark Heiman, Ph.D., and Adrian Noriega, M.D., Ph.D., Pramana Pharmaceuticals Inc. IND readiness for PRM914 a Novel Oral Gut-Brain treatment for Alzheimer’s.
- Judith Kelleher-Andersson, Ph.D., Neuronascent, Inc. Aged CCD Field Trial for Go-no-go Decision of NNI-362 Ph2 POC Trial.
“We are so excited by the possibilities of this new round of Part the Cloud projects,” said Hoag. “The variety of approaches, tools and mechanisms gives us real hope about precision medicine as the future of Alzheimer’s treatment.”
This article may also be found here: https://www.alz.org/news/2026/part-the-cloud-grants-11-million-to-develop-innovative-treatments
To learn more about Part the Cloud, including current and future funding opportunities, visit alz.org/partthecloud.
References:
- Ehrenzweig J, and Hunter RP. 2023. Canine cognitive decline and Alzheimer disease: clinical insights to solve a shared one-health problem. JAVMA Currents in One Health doi.org/10.2460/javma.23.02.0095
- Habiba U, Ozawa M, Chambers JK, Uchida K, Descallar J, Nakayama H, Summers BA, Morley JW and Tayebi M. 2021. Neuronal deposition of amyloid-b oligomers and hyperphosphorylated Tau is closely connected with cognitive dysfunction in aged dogs. J. Alz. Dis. Rep.Vol 5:749-760. DOI 10.3233/ADR-210035.
- Hines AD, McGrath S, Latham AS, Kusick B, Mulligan L, Richards ML and Moreno JA. 2023. Activated gliosis, accumulation of amyloid b, and hyperphosphorylation of tau in aging canines with and without cognitive decline. Frontiers in Aging Neuroscience DOI 10.3389/fnagi.2023.1128521.
- Kandasamy M, RosskopfM, Wagner K, Dlein B, Couillard-Despres S, Reitsamer HA, Stephan M, Nguyen PN, Riess O, Bogdahn U, Winkler J, vonHorsten S and Aigner L. (2015) Reduction in Subventricular zone-derived olfactory bulb neurogenesis in a rat model of Huntington’s disease is accompanied by striatal invasion in neuroblasts. PLOS One.DOI:10.1371/journal.pone.0116069.
- Madari A, Farbakova J, Katina S, Smolek T, Novak P, Weissova T, Noval M and Zilka N. 2015 Assessment of severity and progression of canine cognitive dysfunctions syndrome using CAnine DEmentia Scale (CADES). Applied Animal Behav. Sci. Vol. 171:138-145. doi.org/10.1016/j.applanim.2015.08.034
- Polgar Z, Kinnunen M, Ujvary D, Miklosi A and Gacsi M. 2016. A test of canine olfactory capacity: comparing various dog breeds and wolves in a natural detection task. PLoS ONE 11(5):e0154087.
